FDA Provides Trethera Clearance to Initiate TRE-515 Clinical Trial in Healthy Volunteers to Study Food Effect and Biomarkers
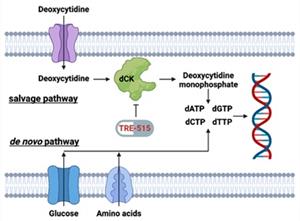
LOS ANGELES, July 30, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, announced today that the U.S. Food and Drug Administration (FDA) has cleared a clinical trial evaluating its novel drug, TRE-515, in healthy volunteers. TRE-515, a clinical stage drug in dose escalation trials for the treatment of solid tumors, targets the enzyme deoxycytidine kinase (dCK) in the deoxyribonucleoside salvage pathway.
The upcoming trial will enroll up to 72 healthy adults and further assess TRE-515 plasma levels and specific predictive biomarkers of the novel drug. The central focus of the study is to evaluate potential food effects on TRE-515 blood levels and the related biomarker deoxycytidine (dC). Oral therapies can be influenced by food intake, potentially altering the absorption of drugs or the activity of biomarkers. Gaining scientifically valid in-depth insights may enable future patients to avoid mandatory fasting periods and enjoy a higher quality of life.
“FDA clearance of our healthy volunteer protocol, combined with promising data from our ongoing oncology trial, underscores the strong safety profile of TRE-515 and positions Trethera to explore its full potential across a broad range of disease conditions,” said Dr. Ken Schultz, Chairman and CEO of Trethera.
The trial will also determine baseline levels of the dC biomarker in a non-diseased population, adding to the most comprehensive human dC biomarker dataset constructed to date. Trethera has already collected close to 1,000 biomarker samples from its ongoing solid tumors trial and will use the same validated assay to compare results with this healthy volunteers trial. Trethera’s biomarker-driven approach supports dose optimization and may enable early identification of patients most likely to respond to therapy, creating a more precise and personalized treatment. Drugs with predictive biomarkers, such as TRE-515, are twice as likely to gain FDA approval compared to drugs without biomarkers.
The FDA clearance not only reinforces TRE-515’s strong scientific rationale but also lays the foundation for the product development path through an FDA drug approval process for cancer and autoimmune indications. Healthy volunteer trials for studying food effects or drug-drug interactions have the advantages of being time efficient and cost effective as well as having better controlled data. Trethera plans to start the trial following dose escalation in its ongoing solid tumors trial.
“Our early effort to identify and integrate TRE-515 biomarkers improves scientific understanding, lowers total trial costs, and creates pathways toward companion diagnostics,” added Dr. Schultz. “This precision approach is foundational to our development strategy and biomarkers have already allowed us to confirm target engagement.”
TRE-515 continues to show clinical promise with a favorable safety and tolerability profile established in oncology patients, exhibiting zero dose limiting toxicities up to 720 mg. Beyond cancer, Trethera continues to actively explore application in inflammatory conditions such as Crohn’s disease, ALS, and lupus.
“TRE-515 represents a departure from the traditional ‘one drug, one disease’ model,” added Dr. Schultz. “The TRE-515 mechanism of action allows for application across multiple diseases, both rare and common, as monotherapy or in combination, which is already reflected in our growing portfolio of FDA designations, including optic neuritis, acute disseminated encephalomyelitis and prostate cancer. Access to healthy volunteer trials provides an advantageous product development path for the FDA drug approval processes in all indications.”
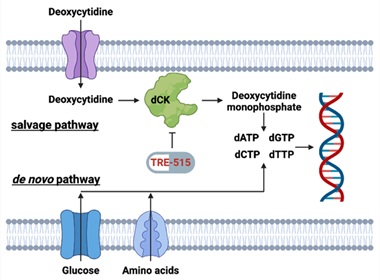
Figure 1: Biochemical pathways for the supply of deoxyribonucleoside triphosphate pools. The salvage pathway becomes upregulated during autoimmune diseases and cancer. TRE-515 blocks the enzyme deoxycytidine kinase (dCK) in the deoxyribonucleoside salvage pathway.
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. The FDA has designated TRE-515 a Fast Track drug for prostate cancer and an Orphan Drug for two autoimmune neurologic diseases. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/197626d8-7002-4305-ae9d-19aef237d28c

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.