Bi-level Positive Airway Pressure (BiPAP) Devices Market Growth to USD$ 3.70 billion by 2033 | Emerging Players (2026)
BiPAP Devices Industry Forecast (2026-2033) | Global Adoption, Revenue Trends & Emerging Players
Bi-level Positive Airway Pressure Devices Market Insights | Advanced Sleep Therapy Growth Trends in United States and Japan”
AUSTIN, TX, UNITED STATES, February 19, 2026 /EINPresswire.com/ -- Market Size and Outlook (2026)— DataM Intelligence 4Market Research LLP
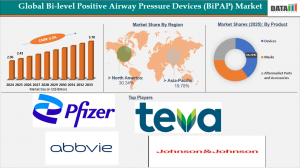
According to DataM Intelligence, the Global Bi-level Positive Airway Pressure (BiPAP) devices market was valued at USD 1.45 billion in 2024, is projected to rise to USD 2.43 billion in 2025, and is expected to reach USD 3.70 billion by 2033, growing at a CAGR of 5.5% between 2026 and 2033
The market expansion is being driven by rising prevalence of sleep apnea, chronic obstructive pulmonary disease (COPD), and other respiratory disorders, combined with increasing adoption of home healthcare solutions and remote patient monitoring systems. Technological advancements, such as smart BiPAP devices with integrated AI, wireless connectivity, and cloud-based monitoring, are improving patient compliance, therapy effectiveness, and clinician oversight.
Download Free Sample PDF Of This Report (Get Higher Priority for Corporate Email ID):– https://www.datamintelligence.com/download-sample/bi-level-positive-airway-pressure-devices-market
FDA Approvals
• August 2025: PMA P240024 – FDA granted Premarket Approval for a respiratory support device (likely bilevel PAP variant) for adults ≥22 years failing CPAP or ineligible for it. Details limited to confirmed CPAP intolerance; intended for OSA therapy.
• 3B Medical Luna G3 Auto‑BiPAP (2021 clearance): FDA 510(k) for OSA with integrated heated tubing and cellular connectivity.
• Somnetics Transcend BiPAP (2020 EUA): Emergency Use Authorization for COVID‑19 respiratory support
New Device Launches by Companies
✅ Several updates and relaunches in 2025–2026 emphasize auto‑adjusting algorithms, connectivity, and CPAP alternatives:
✅ ResMed AirCurve 11 (Feb 2024 U.S. launch; 2025 international expansion): BiLevel device with ASVAuto algorithm (analyzes 13 breath indicators 50x/second) for personalized therapy. Compatible with all ResMed masks; integrates with AirView cloud and myAir app. Compliance improved to 87% with coaching.
✅ Luna G3 BiPAP (3B Medical; ongoing 2025 availability): Highlighted as top 2026 pick; auto‑adjusting with backup rate up to 25 cmH₂O, heated tubing.
✅ No major 2025–2026 launches reported beyond iterative updates (e.g., software for existing models). Market lists ResMed AirCurve BiPAP 10 VAuto and Luna G3 as leading options.
Growth Drivers
1. Over 936 million adults globally were diagnosed with sleep-disordered breathing in 2024, driving device adoption.
2. Remote patient monitoring integration is expected to improve adherence to BiPAP therapy by up to 40% by 2030.
3. Rising awareness of non-invasive ventilation (NIV) therapy among elderly and chronic respiratory patients.
4. Government healthcare initiatives in the U.S., EU, Japan, and India are increasing funding for home respiratory care solutions.
5. COVID-19 aftermath and post-acute respiratory syndrome are increasing BiPAP device usage in rehabilitation and homecare settings.
Regulatory Developments
✦ Ongoing Philips Recall (2021–2026): FDA continues oversight of ventilators, BiPAP, and CPAP due to foam breakdown risks; impacts millions of devices. Philips paused U.S. sales under 2024 consent decree.
✦ PMA P240024 (Aug 2025): Marks recent bilevel respiratory approval, signaling FDA openness to CPAP alternatives for severe OSA
Market Segmentation Analysis
By product segment, BiPAP devices dominated the global market, holding the largest revenue share of 55.71% in 2025.
By Technology / Modality
PROTACs: 42% share in 2024 (USD 1.26B), projected to reach USD 5.8B by 2032
Molecular Glues: 18% share in 2024 (USD 540M), expected to grow to USD 2.7B by 2032
Degronimids: 10% share in 2024 (USD 300M), projected at USD 1.2B by 2032
LYTACs: 6% share in 2024 (USD 180M), expected to reach USD 890M by 2032
AUTACs: 5% share in 2024 (USD 150M), projected at USD 730M by 2032
ATTECs: 4% share in 2024 (USD 120M), expected to grow to USD 610M by 2032
DUB Inhibitors: 5% share in 2024 (USD 150M), projected at USD 700M by 2032
SERD-based Degraders: 3% share in 2024 (USD 90M), expected to reach USD 410M by 2032
BET Degraders: 2% share in 2024 (USD 60M), projected at USD 280M by 2032
Others: 5% share in 2024 (USD 150M), expected to grow to USD 740M by 2032
By Target Protein Class
Transcription Factors: 28% share in 2024 (USD 840M), projected to reach USD 3.4B by 2032
Nuclear Hormone Receptors: 20% share in 2024 (USD 600M), expected to grow to USD 2.5B by 2032
Epigenetic Regulators: 15% share in 2024 (USD 450M), projected at USD 1.9B by 2032
Kinases: 12% share in 2024 (USD 360M), expected to reach USD 1.6B by 2032
Scaffold Proteins: 8% share in 2024 (USD 240M), projected at USD 1.1B by 2032
Misfolded/Aggregated Proteins: 10% share in 2024 (USD 300M), expected to grow to USD 1.3B by 2032
Others: 7% share in 2024 (USD 210M), projected at USD 920M by 2032
By Therapeutic Area
Oncology: 45% share in 2024 (USD 1.35B), projected to reach USD 6.2B by 2032
Immunology / Inflammatory: 15% share in 2024 (USD 450M), expected to grow to USD 2B by 2032
Neurology: 10% share in 2024 (USD 300M), projected at USD 1.3B by 2032
Ophthalmology: 5% share in 2024 (USD 150M), expected to reach USD 660M by 2032
Respiratory: 7% share in 2024 (USD 210M), projected at USD 900M by 2032
Infectious Diseases: 8% share in 2024 (USD 240M), expected to grow to USD 1.1B by 2032
Rare / Genetic Disorders: 5% share in 2024 (USD 150M), projected at USD 700M by 2032
Metabolic Disorders: 3% share in 2024 (USD 90M), expected to reach USD 420M by 2032
Others: 2% share in 2024 (USD 60M), projected at USD 280M by 2032
By Route of Administration
Oral: 50% share in 2024 (USD 1.5B), projected to reach USD 6.5B by 2032
IV (Intravenous): 25% share in 2024 (USD 750M), expected to grow to USD 3.2B by 2032
SC (Subcutaneous): 15% share in 2024 (USD 450M), projected at USD 2B by 2032
Intravitreal: 5% share in 2024 (USD 150M), expected to reach USD 700M by 2032
Others: 5% share in 2024 (USD 150M), projected at USD 690M by 2032
By Stage of Development
Discovery: 20% share in 2024 (USD 600M), projected to reach USD 2.5B by 2032
Preclinical: 15% share in 2024 (USD 450M), expected to grow to USD 1.9B by 2032
Clinical Trials (Phase I–III): 40% share in 2024 (USD 1.2B), projected at USD 5.3B by 2032
Approved / Commercialized: 25% share in 2024 (USD 750M), expected to reach USD 3.2B by 2032
By End User
Hospitals: 35% share in 2024 (USD 1.05B), projected to reach USD 4.6B by 2032
Clinics: 20% share in 2024 (USD 600M), expected to grow to USD 2.5B by 2032
Research / Academic Labs: 15% share in 2024 (USD 450M), projected at USD 1.9B by 2032
Pharma / Biotech: 20% share in 2024 (USD 600M), expected to reach USD 2.7B by 2032
CROs: 10% share in 2024 (USD 300M), projected at USD 1.3B by 2032
By Age Group
Children & Adolescents: 10% share in 2024 (USD 300M), projected to reach USD 1.3B by 2032
Adults: 65% share in 2024 (USD 1.95B), expected to grow to USD 8B by 2032
Geriatric: 25% share in 2024 (USD 750M), projected at USD 3.2B by 2032
By Degradation Pathway
Ubiquitin–Proteasome System (UPS): 45% share in 2024 (USD 1.35B), projected to reach USD 6B by 2032
Autophagy–Lysosome: 25% share in 2024 (USD 750M), expected to grow to USD 3.2B by 2032
Endosome–Lysosome: 10% share in 2024 (USD 300M), projected at USD 1.3B by 2032
Chaperone-Mediated: 5% share in 2024 (USD 150M), expected to reach USD 660M by 2032
Others: 15% share in 2024 (USD 450M), projected at USD 2B by 2032
By Protein Localization
Nuclear: 30% share in 2024 (USD 900M), projected to reach USD 4B by 2032
Cytoplasmic: 25% share in 2024 (USD 750M), expected to grow to USD 3.2B by 2032
Membrane-Bound: 20% share in 2024 (USD 600M), projected at USD 2.7B by 2032
Extracellular: 15% share in 2024 (USD 450M), expected to reach USD 2B by 2032
Others: 10% share in 2024 (USD 300M), projected at USD 1.3B by 2032
Request for Customized Sample Report as per Your Business Requirement:- https://www.datamintelligence.com/customize/bi-level-positive-airway-pressure-devices-market
By Region
North America
North America dominates the global Bi-level Positive Airway Pressure (BiPAP) devices market, accounting for the largest revenue share of 30.34% in 2025.
High adoption in the U.S. due to rising sleep apnea prevalence and reimbursement coverage for home NIV therapy.
Europe
Market size: USD 412M (2024) → USD 1.48B (2032)
Germany, UK, and France lead adoption; aging population and robust homecare policies drive demand.
Asia-Pacific
Market size: USD 283M (2024) → USD 940M (2032)
Japan, China, and India show fastest CAGR; rising awareness, increasing chronic respiratory disorders, and growing healthcare infrastructure.
Rest of the World (RoW)
Market size: USD 143M (2024) → USD 480M (2032)
Middle East and Latin America witnessing gradual adoption in private hospitals and homecare setups.
Competitive Landscape
The BiPAP devices market is moderately consolidated with major medical device companies and emerging healthcare startups driving innovation in smart therapy, patient comfort, and telehealth integration.
Key Players
1. Philips Respironics
2. ResMed Inc.
3. Fisher & Paykel Healthcare
4. DeVilbiss Healthcare
5. Drive DeVilbiss Healthcare
6. BMC Medical
7. Invacare Corporation
8. Somnetics International
Key Highlights:
✅ Philips Respironics launched AI-driven BiPAP devices with cloud-based adherence tracking (Jan 2025).
✅ ResMed’s AirSense 11 ST model reported 18% YoY adoption growth across homecare settings (Feb 2025).
✅ Fisher & Paykel introduced compact portable BiPAP with auto-adjusting pressure for travel patients (Mar 2025).
✅ BMC Medical expanded distribution in Asia-Pacific, reaching 120,000 units in 2024.
Recent Developments
🔹 Philips Respironics partnered with telehealth providers to integrate BiPAP devices into remote monitoring platforms (April 2025).
🔹 ResMed acquired an AI start-up for predictive respiratory therapy adherence analytics (Feb 2025).
🔹 Drive DeVilbiss Healthcare launched eco-friendly, low-noise BiPAP devices for homecare (Jan 2025).
Buy Now & Unlock 360° Market Intelligence:- https://www.datamintelligence.com/buy-now-page?report=bi-level-positive-airway-pressure-devices-market
Market Outlook & Opportunities
1. Homecare BiPAP segment projected to exceed USD 2.5B by 2032, driven by telemedicine and patient-centric therapy.
2. Asia-Pacific expected to record fastest CAGR (15%), driven by rising awareness, growing healthcare budgets, and chronic respiratory disorders.
3. Integration with IoT, AI, and cloud platforms can unlock USD 600M additional value by 2032.
4. Smart BiPAP devices with automated pressure adjustment and real-time adherence monitoring expected to become standard in chronic respiratory care by 2030.
Conclusion
The Global Bi-level Positive Airway Pressure Devices Market is witnessing a transformation from conventional respiratory therap, BiPAP devices are becoming essential in managing sleep apnea, COPD, and chronic respiratory diseases.
With technological innovation, telehealth integration, and regulatory support, BiPAP devices are redefining non-invasive ventilation, improving patient adherence, and reducing hospitalization rates globally.
Related Reports
Positive Airway Pressure (PAP) Devices Market
Continuous Positive Airway Pressure (CPAP) Devices Market
Sai Kiran
DataM Intelligence 4market Research LLP
+1 877-441-4866
sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
YouTube
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.